Decontaminate N95 and/or FFP2 Respirators with Bioquell Technology
N95 and/or FFP2 respirators are a vital component in protecting frontline healthcare professionals. They are a necessary part of treating patients infected with COVID-19, but supplies have been limited due to the pandemic. Healthcare facilities can now reprocess and reuse respirators up to 20 times with Bioquell technology.
Contact Us for Details Download Overview
REPROCESS N95 and/or FFP2 RESPIRATORS IN YOUR OWN HOSPITAL NOW
Health Sciences Authority (HSA) has issued a provisional authorisation for the emergency use of Ecolab’s Bioquell Technology for use in decontaminating compatible N95 and/or FFP2 Respirators, for reuse by healthcare personnel.
Each mask can be decontaminated up to 20 times. Additionally, the Bioquell process is residue-free, automated and proven with over 50 peer-reviewed studies.
For other solutions during COVID-19, please visit: https://www.ecolab.com/pages/coronavirus
How does it work?
With Bioquell technology, every exposed surface in an enclosed area is covered in a micro-condensation of hydrogen peroxide vapour, effectively killing organisms it comes in to contact with while retaining the integrity of the mask.

Regulatory Details
The Bioquell process (35% aqueous hydrogen peroxide + Bioquell technology) is a registered sterilant. A complete review of over 50 peer-reviewed studies connected to Bioquell’s efficacy can be found here.

Bioquell’s History with Dangerous and Emerging Pathogens
From SARS in 2003 to Ebola in 2014 to MERS-CoV in 2017, Bioquell technology has been deployed worldwide in the battle against novel coronaviruses and other virulent pathogens.
Bioquell N95 and/or FFP2 Respirator Decontamination Information
Overview and Reports
How the Bioquell Process Works
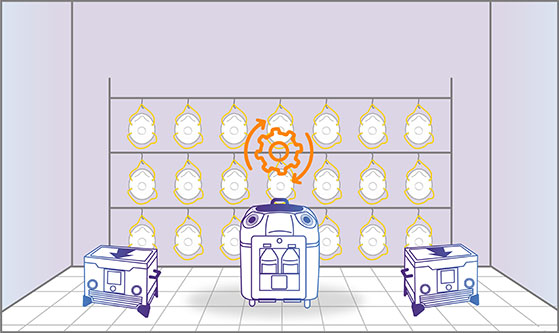
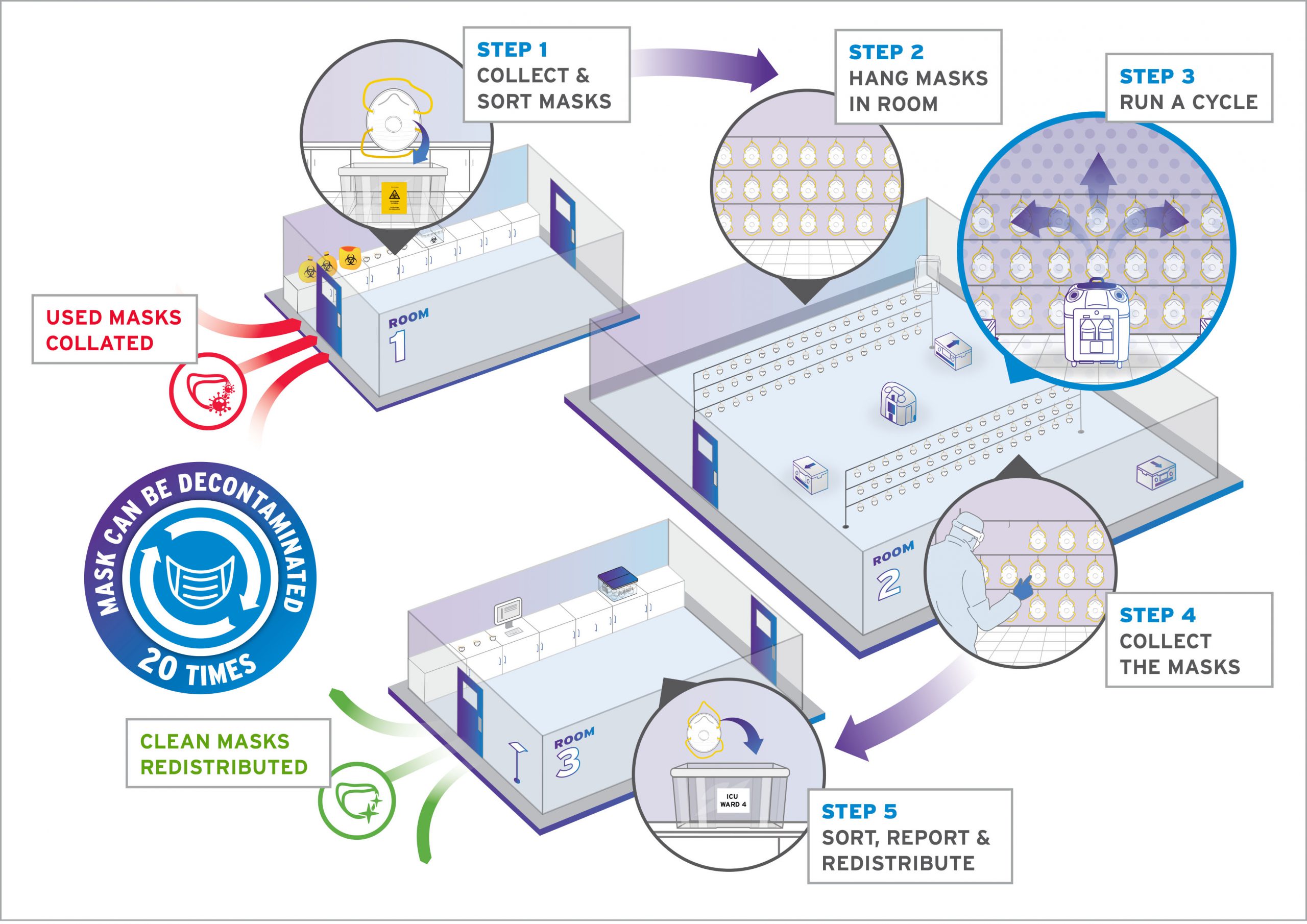
Step 1: Preparation
The Bioquell decontamination system warms up, getting ready to disperse the vapour. You do not need to reach or wait for temperature or humidity levels to begin.
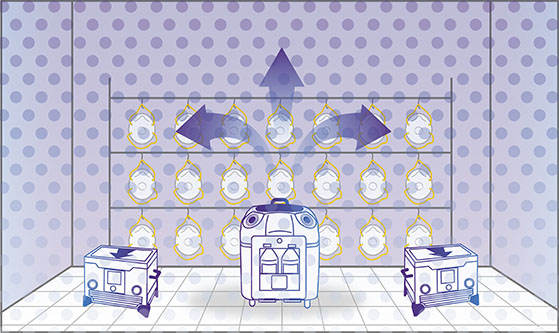
Step 2: Vaporization
The Bioquell decontamination system emits the vapour into the enclosed area and fills the space, pushing the vapour against every exposed surface, including surrounding complex shapes and crevices.
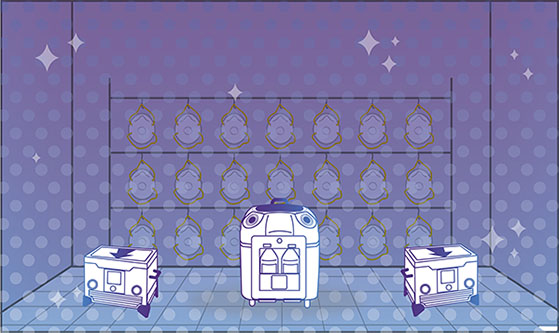
Step 3: Dwell
With vaporisation complete, the enclosed area is at a standstill, allowing the peroxide to dwell on every exposed surface and kill organisms.
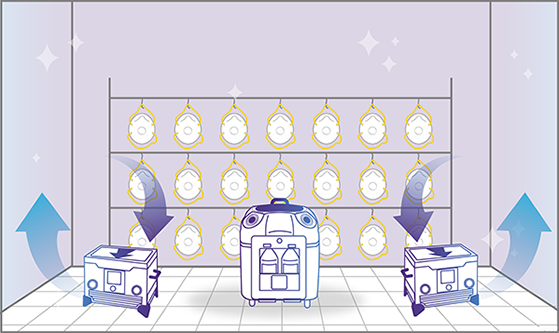
Step 4: Aeration
Your HVAC system, high-powered Bioquell aeration units using catalytic conversion or a combination of both now safely remove all of the hydrogen peroxide vapour from the enclosed area. When aerated with Bioquell units, the vapour is converted into water vapour and oxygen.
Recommended Bioquell Systems
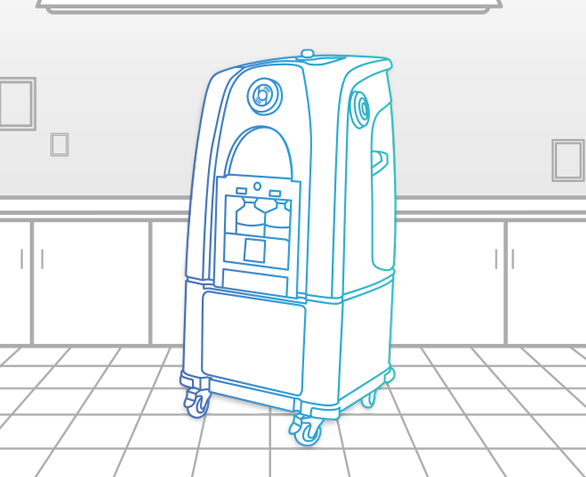
Bioquell ProteQ
Quickly and effectively decontaminate nearly any room or zone with this mobile, scalable and compliant system with wireless communication technology. All components, including built-in aeration and room for additional optional aeration, are hosted within its frame, making setup simple.
Ideal for:
- Biopharmaceutical Manufacturing Areas
- Production Labs
- Room and Zone Decontamination
- Bio-Safety Labs
- Cleanrooms
- GMP/GLP Laboratories
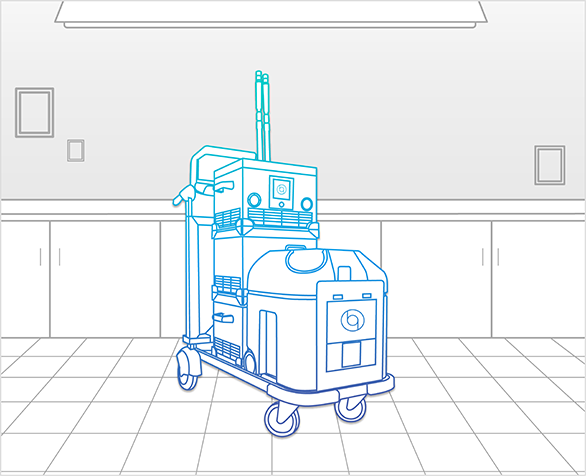
Bioquell BQ-50
Decontaminate patient rooms and small spaces in your facility with Ecolab’s mobile, robust and easy-to-use system: the Bioquell BQ-50. The latest advancement including increased aeration capabilities and Direct Cycle Control (DCC).
Ideal for:
- Killing organisms
- Outbreak response
- Patient rooms
- Critical care areas
- Operating rooms
- Emergency response preparedness
Latest News
See below for some of the latest news regarding Bioquell technology and N95 respirator decontamination
MetroHealth sterilizing 50,000 N95 masks per day amid coronavirus pandemic
Doylestown Hospital has a sterile solution to the N95 mask shortage
Health reporter Haley Hernandez shows how healthcare workers are reusing masks and how you can too
Media inquiries
Please submit all media inquiries to bioquellusorders@ecolab.com
Important Information
The Bioquell Technology System has neither been cleared nor approved for the indication to treat patients with COVID-19 infection.
The Bioquell Technology System has been authorized by Health Canada under the Interim Order.
The Bioquell Technology System is authorized only for the duration of the COVID-19 pandemic declaration, unless the authorization is terminated or revoked sooner.
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Ecolab Inc
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
bioquellusorders@ecolab.com