Vein to Vein compliance with
Ecolab Cell and Gene Therapy Solutions
Discover the complete contamination control strategy for your company.
From R&D to clinical trial to production, partner with Ecolab to keep contamination risk under control and your operations on track.
Watch Video |Download The EBook
|Download The EBook
ALWAYS PLAN WITH REGULATORY COMPLIANCE IN MIND

Cell and Gene Therapy industry provides a unique regulatory challenge to navigate. Compliance very often relies on a case by case risk assessment.
Ideal for Laboratory to GMP Cleanroom
Regulation trends reflected in the recent EU GMP Annex 1 update formally recommend to use of closed system as Isolators. Such enclosures give access to a better in-process contamination control. It should be implemented early in development phase to ease transition from R&D to clinical trials and commercial manufacturing.
The development of novel Cell & Gene therapies such as CAR T-Cells, mRNA or stem cell therapy requires extended Research and Development activity before starting clinical trials. Regulators recommend to find a closed containment system ]that offers the least invasive option while retaining an aseptic space for you to work within. This would mean no building work, no HVAC connections needed, and no additional electrical work to use the device.
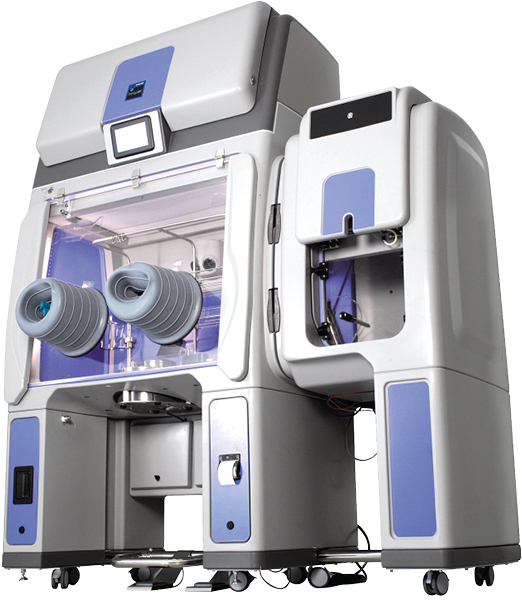
ECOLAB’S BIOQUELL QUBE ISOLATOR, THE IDEAL SOLUTION

“*EPA Registration Number: 72372-1-86703”
ADVANTAGES OF THE BIOQUELL QUBE ISOLATOR

SPEED
- Quick implementation and short lead time
- Fast bio-decontamination cycle

CONSISTENCY
- Create a Grade A / ISO 5 area with validated and automated biodecontamination cycle for repeatable results
COST
- Cost effective option or scalable and effective work environments

FLEXIBILITY & SCALABILITY
- Ability to expand the working space as your operations grow

COMPLIANCE
- Helps provide product consistency to assist in meeting compliance standards from regulatory bodies around the world
SEE THE SAVINGS
Download our case studies
Showcasing the Bioquell Qube’s impact in cell and gene therapy.
Case Study 1: See how this company saved hundreds of hours in productivity and cut energy costs.
Case Study 2: Discover how a company increase profitability while reducing batch failure and more.

VIEW THE CONFIGURATIONS
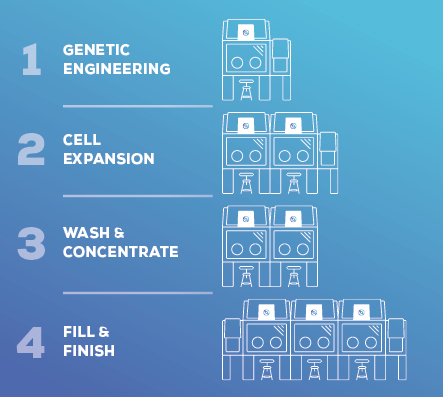
In EU GMP Annex 1, and is a likely recommendation for an appropriate Contamination Control Strategy (CCS) to ensure compliance and patient safety. Isolator systems can create a closed process that helps mitigate the risk of contamination during all critical operations from genetic engineering to fill/finish.
The Bioquell Qubes modularity supports your operations, from small batch production to steps with multiple human interactions to regulatory and quality control needs.
COMPREHENSIVE SOLUTIONS FOR YOUR CLEANING AND DISINFECTION NEEDS

Cleaning and Disinfection Solutions
- Bio-decontamination equipment for rooms, equipment, pass-throughs, material airlocks and more

Integrated Bio-decontamination Isolators
- Bio-decontamination service option for a fully managed, scalable response for your scheduled or emergency response needs
- The Bioquell Qube isolator
- Manual cleaning and disinfection

Validation Support
- Support to validate your Contamination Control Strategy according to regulation and avoid findings during audits
MORE THAN JUST CLEANING AND DISINFECTION EQUIPMENT AND SUPPLIES
- Find operational efficiencies
- Increase the total value of your process by sticking with GMP and other regulatory guidance
- Create compliance-based SOPs early for faster approvals
- Discover energy and other cost savings
- Ensure your cleaning and disinfection products are compatible with your operations
- Focus on building lifesaving products, while we handle contamination control

Learn more about Bioquell’s technology here or contact us below
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Ecolab Inc
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
bioquellusorders@ecolab.com






